DT-J-ASC : Head Plate Type Jacket Container with Stand
Features
The container and frame can be separated.
Head plate bottom type with temperature control jacket and excellent agitation and discharge properties
○The container and frame can be separated and fixed according to the work without tools.
○Since the container can be removed from the stand and washed, the washing time can be shortened.
○The container and frame are made of durable and rust-resistant SUS 304.
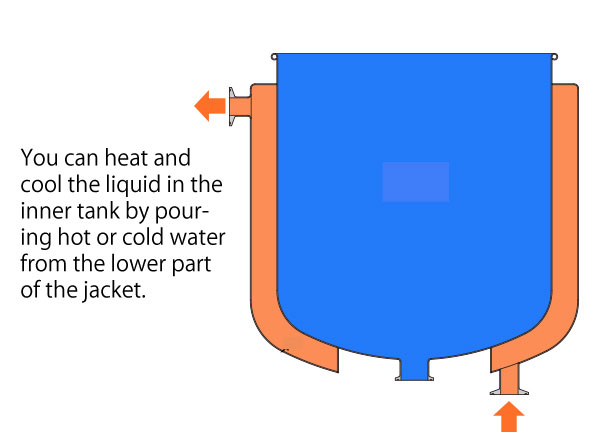
○Comes with a jacket (outer cistern) that can keep the contents warm and cool by flowing water.
○The 150 L container has metal fittings (holes) for lifting.
○It is possible to make only the container. ([DT-J-BRK])
Price:
Product Specifications
Container Body/Lid/Handle ... SUS 304
Lever Band (DT-CTL-J-ASC only) ... SUS 304
Packing (DT-CTL-J-ASC only) ... Silicon Rubber (Food Sanitation Law Compliant Product)
Caster Material ... SUS + Urethane Truck (With Inner Stopper 2 Pieces)
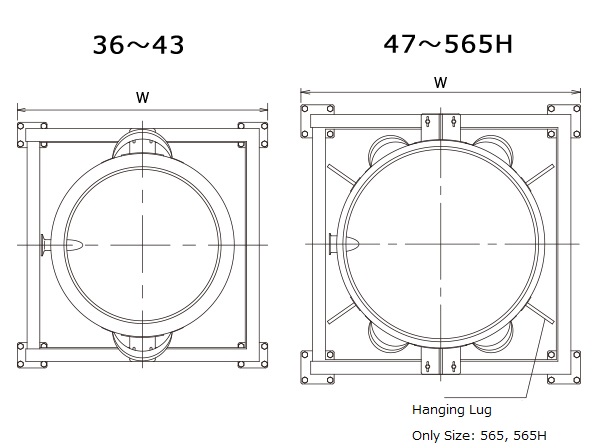
Hanging Lug (565 size only) ... SUS 304
Jacket Nozzle ... 1S Ferrule
●Surface Treatment: Inner and Outer Surface 0.2Ra
●Conditions of use: atmospheric pressure (Do not pressurize or decompress.)
*Please contact us if you want to put pressure on the jacket.
Capacity: 35 L, 45 L, 60 L, 80 L, 100 L, 150 L
*Other capacities are also available.
*Bracket specifications vary depending on size.
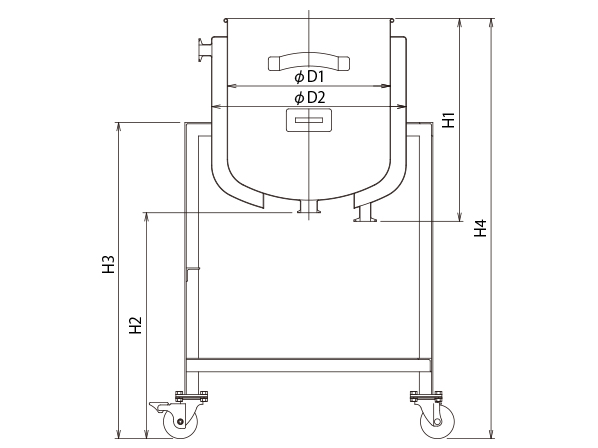
Reference Dimensional Drawing
Drawings & Certificates
Request Drawings & Certificates / Contact UsDetails & Notes
| Model | Capacity | Weight | Diameter | Diameter | Container Height | Drain Height | Stand Height | Overall Height | Stand Width | Drain | Load Bearing Capacity | Lead Time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | kg | D1 | D2 | H1 | H2 | H3 | H4 | W | Size | kg | Days | |
| DT-ST-J-ASC-36 | 35 | 24 | 360 | 430 | 449 | 500 | 700 | 930 | 590 | 1.5S | 259 | 35 |
| DT-ST-J-ASC-39 | 45 | 25 | 387 | 430 | 489 | 500 | 700 | 970 | 590 | 1.5S | 259 | 35 |
| DT-ST-J-ASC-43 | 60 | 42 | 430 | 470 | 520 | 500 | 700 | 1005 | 650 | 2S | 390 | 35 |
| DT-ST-J-ASC-47 | 80 | 53 | 470 | 565 | 577 | 500 | 750 | 1055 | 750 | 2S | 390 | 35 |
| DT-ST-J-ASC-47H | 100 | 57 | 470 | 565 | 692 | 500 | 750 | 1170 | 750 | 2S | 390 | 35 |
| DT-ST-J-ASC-565 | 150 | 75 | 565 | 635 | 717 | 500 | 800 | 1195 | 860 | 2S | 489 | 40 |
| Model | Capacity | Weight | Diameter | Diameter | Container Height | Drain Height | Stand Height | Overall Height | Stand Width | Drain | Load Bearing Capacity | Lead Time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | kg | D1 | D2 | H1 | H2 | H3 | H4 | W | Size | kg | Days | |
| DT-CTL-J-ASC-36 | 35 | 25 | 360 | 430 | 449 | 500 | 700 | 930 | 590 | 1.5S | 259 | 35 |
| DT-CTL-J-ASC-39 | 45 | 26 | 387 | 430 | 489 | 500 | 700 | 970 | 590 | 1.5S | 259 | 35 |
| DT-CTL-J-ASC-43 | 60 | 43 | 430 | 470 | 520 | 500 | 700 | 1005 | 650 | 2S | 390 | 35 |
| DT-CTL-J-ASC-47 | 80 | 54 | 470 | 565 | 577 | 500 | 750 | 1055 | 750 | 2S | 390 | 35 |
| DT-CTL-J-ASC-47H | 100 | 58 | 470 | 565 | 692 | 500 | 750 | 1170 | 750 | 2S | 390 | 35 |
| DT-CTL-J-ASC-565 | 150 | 77 | 565 | 635 | 717 | 500 | 800 | 1195 | 860 | 2S | 489 | 40 |

Available to products made of SUS316L material!
The standard material for Nitto Kinzoku is SUS304. SUS316L is a material with higher corrosion resistance than SUS304. It contains an ingredient called “molybdenum”, which is not contained in SUS304. This molybdenum makes the passive film thicker and improves the corrosion resistance. SUS316L is a low-carbon type stainless steel with a low carbon content, and is resistant to corrosion (intergranular corrosion) in the welding area where parts are connected to each other. SUS316L is generally recognized as a high-grade model of stainless steel material. It is a material that is commonly used in production sites in the pharmaceutical industry. If you are concerned about rust and corrosion resistance, we recommend you to select SUS316L. For more details, please contact us.Q&A
- Q Can you make a clip type (HT-CTH-J-ASC)?
- AIt can be manufactured, but the height of the jacket portion than convenience on the ST / CTL of the clip will be lower.
- QIs it possible to customize the frame and container?
- AChanging the height, or you can attach the parts. Please tell us the specifications of your choice.
- QWe want to lift with a forklift the container, but ...
- AWe will attach the bracket for the forklift.
- QOr stand separation type of pressurized container can be manufactured?
- AIt is possible to manufacture. Please tell us the specifications of your choice.
- QOr container and the frame is there anything that is in one piece?
- AThere is a container of the caster with legs (DT-J-L).